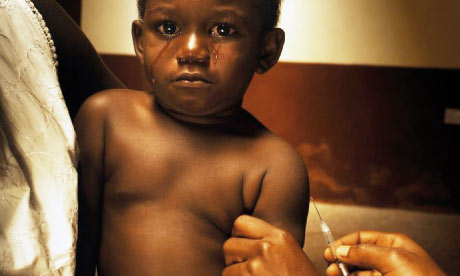
More than 25,000 participants and 2,000 journalists from approximately 200 countries are expected to convene at the conference, which is predicted to be a landmark event in the history of HIV and AIDS both in the United States and globally.
“In the last couple of years we have seen some incredibly important breakthroughs in science,” said. Elly Katabira, International Chair of AIDS 2012 and President of the International AIDS Society (IAS). “The results of the CAPRISA trial presented at AIDS 2010 and the data from IAS 2011 proving beyond a doubt that treatment is prevention have shown us that we now have the real potential to change the direction of HIV. Science has provided us with the tools, what we need now is a global political and economic commitment to action. A turbulent economic climate must not halt funding for research and implementation”.
The return of the International AIDS Conference to the United States after more than 20 years represents a victory for public health and human rights and it is the result of dedicated advocacy to end the nation’s misguided entry restrictions on people living with HIV.
“Hosting AIDS 2012 in the U.S will be an occasion to highlight the disparities in access to treatment and care which exist in the country.” said Diane Havlir, Local Co-Chair of AIDS 2012. “We hope for a broad and active participation from all of those affected by the HIV epidemic, particularly people living with HIV and AIDS, policy-makers and key affected populations”.
Together with delegate and media registration, 1 December is the opening day for online abstract submissions. Over half of all conference sessions will be abstract-driven and all of the submissions will go through an extensive peer-reviewed process in order to guarantee the highest caliber of state-of-the-art science. The online abstract submission closes on 15 February 2012 and reopens on 19 April 2012 for late breaker abstract submissions.
In addition to the conference sessions, AIDS 2012 will feature a set of workshops open to delegates. Workshops will fall under professional development, community skills and leadership skills. Online submissions for workshops open on 1 December 2011 and close on 15 February 2012.
AIDS 2012 will host a Global Village, open to conference delegates and the general public, aimed at intensifying the involvement of key affected populations and other stakeholders in the conference. The conference also presents a Youth Programme with the goal of strengthening the participation of young people and youth issues in the conference through activities such as youth-driven sessions. From today it is possible to submit applications for both the Global Village and the Youth Programme. Both the submissions close on 15 February 2012.
With over 7,000 square metres of prime exhibition space AIDS 2012 offers both commercial and non-commercial organizations the opportunity to showcase their products and services to a wide public. Exhibitor applications open on December 1 and close on 25 May 2012. Exhibition space is limited so early bookings are strongly encouraged.
Various satellite meetings will take place in the conference centre during AIDS 2012. These satellite meetings are fully organized and coordinated by the organization hosting the satellite. The satellite slots are available for a fee and the applications open on 1 December and close on 31 March 2012.
From 8 December online applications for scholarships will be open. The International and Media Scholarship Programme is open to everyone around the world and is aimed at making the conference accessible to people from resource-limited settings and communities. Priority is given to those whose participation will help enhance their work in their own communities. A limited number of scholarships will be also available for media representatives.
More information on registration process and registration fees is available here: http://www.aids2012.org/Default.aspx?pageId=368
[Content that is linked from other sources is for informational purposes and should not construe a Mapping Pathways position.]